PhD student Peter Krogstrup from the Nano-Science Center at the Niels Bohr Institute, University of Copenhagen, developed a theoretical model to explain why and how nanocrystal wires (nanowires) form, in collaboration with researchers from CINAM-CNRS in Marseille. The results have been published in the scientific magazine, Physical Review Letters.
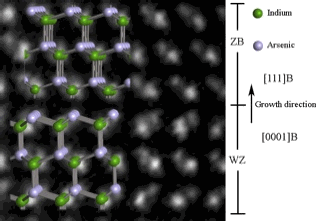 |
| High-resolution image of the crystal structure of an InAs nanowire photographed with an electron microscope. The smallest distance between the indium and arsenic atoms seen in the picture (illustrated with green and gray), is 15 millionths of a millimeter. The nanowire is grown in the direction of the arrow. During growth the crystal structure of the nanowire changes from being hexagonal (WZ) to cubic (ZB). From the crystal orientation seen in the image, the hexagonal structure is characterized by the direction from the In to As atoms changes from layer to layer, while the direction of the cubic structure is always the same. |
Krogstrup laid the foundations for a greater understanding of nanowires and potentially improving their performance, which could benefit solar cells and electronics. In the latest edition of Physical Review Letters, he describes how, under certain conditions, nanowires form a crystal structure that were not expected to be possible. See the article here: http://prl.aps.org/abstract/PRL/v106/i12/e125505
"Crystals will always try to take the form in which their internal energy is as little as possible. It is a basic law of physics and according to it these nanowires should have a cubic crystal structure, but we almost always see that a large part of the structure is hexagonal," explains Krogstrup.
He has, as part of his doctoral dissertation, examined the shape of the catalyst particle (a little nano-droplet), which controls the growth of the nanowires. It appears that the shape of the droplet depends on the amount of atoms from group 3 in the periodic system, which make up half of the atoms in the nanowire crystal. The other half, atoms from group 5 in the periodic system, are absorbed by the drop. The atoms organize themselves into a lattice, and the nanowire crystal grows.
"We have shown that it is the shape of the droplet that determines what kind of crystal structure the nanowires obtain and with this knowledge it will be easier to improve the properties of the nanowires," explains Krogstrup. "The crystal structure has an enormous influence on the electrical and optical properties of the nanowires and you would typically want them to have a certain structure, either cubic or hexagonal. The better nanowires we can make the better electronic components we can make to the benefit of us all."
Krogstrup’s research is conducted in collaboration with the firm SunFlake A/S, which is located at the Nano-Science Center at the Niels Bohr Institute, University of Copenhagen. The company is working to develop solar cells of the future based on nanowires.
Learn more at http://www.nbi.ku.dk/english/
Follow Small Times on Twitter.com by clicking www.twitter.com/smalltimes. Or join our Facebook group

