April 23, 2011 — Virginia Commonwealth University researchers have identified the molecular mechanisms at play for the non-additive wetting free energies at chemically heterogeneous surfaces.
Across disciplines, researchers have long wondered how best to predict hydrophobicity — a molecule’s resistance to wetting — of a mixed surface from the knowledge of its pure constituents. These findings provide insight into that basic physical phenomenon and can be used to predict and understand the energy cost to wet a surface, which may be applied to nanochemistry, materials science and biophysics. The study was published in the April 1 issue of the Proceedings of the National Academy of Sciences (http://www.pnas.org/content/108/16/6374.short).
 |
|
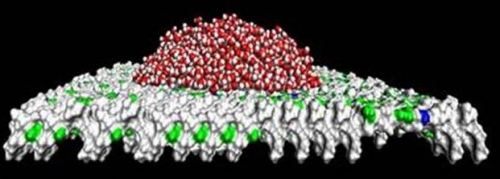
Image. Experiments in silico uncover molecular mechanisms of surface wetting: nanodroplet geometry used in calculation of water contact angle on flattened melittin, a natural nanopatterned surface. Image courtesy of Alenka Luzar, Ph.D.,/VCU.Sathya Achia Abraham, VCU Communications and Public Relations. |
"The work addresses, via molecular-level simulations, a routine feature of macroscopic surface measurements done daily in experimental labs and shows that the observed results can be interpreted quite straightforwardly when the molecular scale features of surface topography, and a little chemistry, are considered," said principal investigator Alenka Luzar, Ph.D., a professor in the VCU Department of Chemistry.
According to Luzar, understanding the wetting of nanopatterned surfaces at a molecular level are important for everyday applications in materials science (inkjet printing, for example), and biology in protein hydration.
"Solvent access to distinct surface ingredients, in addition to the chemical composition, is key to the wettability of a heterogeneous material. This notion can guide computer-assisted design (CAD) of improved materials and helps explain the solubility and function of biomolecules in aqueous solution," she said.
The team demonstrated non-additivity of the cosine of contact angle, or the adhesion energy, on the fraction of hydrophobic/hydrophilic chemistries by measuring nano-droplet contact angles on model surfaces. They found these deviations to be positive, meaning the surface is more hydrophilic at a given fraction than previously predicted by linear expectation, or negative, meaning that the surface is more hydrophobic than previously predicted. Luzar said that the source of these effects can be traced down to size-asymmetry of distinct surface functionalities, as the more prominent groups hide the smaller ones.
Jihang Wang, Ph.D., a graduate student in Luzar’s research group, is the lead author on this paper, which is based on work done solely at VCU. Luzar also collaborated with Dusan Bratko, Ph.D., a professor with the VCU Department of Chemistry.
The study was supported in part by the National Science Foundation and U.S. Department of Energy.
Learn more at http://www.vcu.edu/
Also read: Accurate assessment of water quality for improved process control
Follow Small Times on Twitter.com by clicking www.twitter.com/smalltimes. Or join our Facebook group

