May 11, 2011 — An international research team has discovered a new method to produce belts of graphene called nanoribbons. Using hydrogen, they have unzipped single-walled carbon nanotubes (SWCNT). The method also opens the road for producing nanoribbons of graphane, a modified and promising version of graphene.
As a conductor of electricity, graphene performs as well as copper. As a conductor of heat, it outperforms all other known materials. Variations of graphene properties could be acheived by making graphene in "belts" with various widths (nanoribbons). Nanoribbons were prepared for the first time two years ago, and can be produced by using oxygen treatment to unzip carbon nanotubes. However, this method leaves oxygen atoms on the edges of nanoribbons.
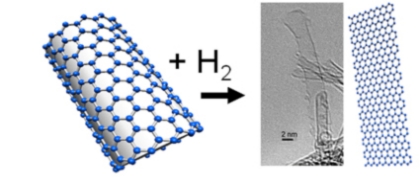 |
| Figure. Researchers unzipped single-walled carbon nanotubes by using a reaction with molecular hydrogen. |
In the new study, the research team shows that it is also possible to unzip single-walled carbon nanotubes by using a reaction with molecular hydrogen. Nanoribbons produced by the new method will have hydrogen on  the edges, which can be an advantage for some applications. Alexandr Talyzin (pictured at left), physicist at Umeå University in Sweden, has over the past decade been studying how hydrogen reacts with fullerenes.
the edges, which can be an advantage for some applications. Alexandr Talyzin (pictured at left), physicist at Umeå University in Sweden, has over the past decade been studying how hydrogen reacts with fullerenes.
"Treating the carbon nanotubes with hydrogen was a logical extension of our research. Our previous experience has been of great help in this work," says Alexandr Talyzin.
Nanotubes are typically closed by semi-spherical cups, essentially halves of fullerene molecules. The researchers have previously proved that fullerene molecules can be completely destroyed by very strong hydrogenation. Therefore, they expected similar results for nanotube end cups and tried to open the nanotubes via hydrogenation. The effect was confirmed, and additional effects came to light.
Some carbon nanotubes were unzipped into graphene nanoribbons as a result of prolonged hydrogen treatment. Unzipped graphene ribbons with hydrogen attached to the side walls could possibly lead to synthesis of hydrogenated graphene: graphane. So far, graphane synthesis was mostly attempted by reacting hydrogen with graphene. This appeared to be very difficult, especially if the graphene is supported on some substrate and only one side is available for the reaction. However, hydrogen reacts more easily with the curved surface of CNTs.
Ilya V. Anoshkin, Albert G. Nasibulin, Jiang Hua and Esko I. Kauppinen at Aalto University are experts in the synthesis and characterization of singled-walled carbon nanotubes. Valery M. Mikoushkin, Vladimir V. Shnitov and Dmitry E. Marchenko from St. Petersburg made XPS and other characterization using synchrotron radiation. Dag Noréus at Stockholm University shared his expertise with high temperature hydrogen reactors.
The research was published in the journal ACS Nano:
Title: Hydrogenation, Purification, and Unzipping of Carbon Nanotubes by Reaction with Molecular Hydrogen: Road to Graphane Nanoribbons
Authors: Alexandr V. Talyzin, Serhiy Luzan, Ilya V. Anoshkin, Albert G. Nasibulin, Hua Jiang, Esko I. Kauppinen, Valery M. Mikoushkin, Vladimir V. Shnitov, Dmitry E. Marchenko, and Dag Noréus
Access it at http://pubs.acs.org/doi/abs/10.1021/nn201224k
Follow Small Times on Twitter.com by clicking www.twitter.com/smalltimes. Or join our Facebook group

